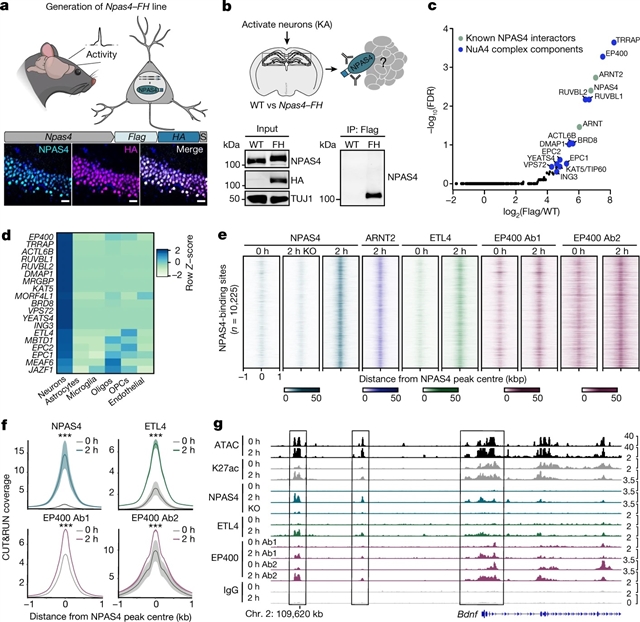
美国哈佛医学院Michael E. Greenberg小组发现,NPAS4–NuA4复合物偶联突触活性与DNA修复。相关论文于2023年2月15日在线发表于国际学术期刊《自然》。
研究人员人员发现了一种活性依赖的DNA修复机制,其中一种新形式的NuA4-TIP60染色质修饰复合物在激活的神经元中围绕诱导的神经元特异性转录因子NPAS4组装。研究人员从大脑中纯化了这种复合物,并证明了它在诱导神经元转录组和回路的活动依赖变化方面的功能。通过描述大脑中活动诱导的DNA双链断裂的景观,研究人员表明NPAS4-NuA4与周期性受损的调控元件结合,并招募额外的DNA修复机制来刺激它们的修复。由NPAS4-NuA4结合的基因调控元件部分地防止年龄依赖性的体细胞突变积累。受损的NPAS4-NuA4信号会导致一系列细胞缺陷,包括活性依赖的转录反应失调,对神经元抑制的失控和基因组不稳定,这些都最终导致有机体寿命缩短。此外,在NuA4复合体的几个组成部分的突变会导致神经发育和自闭症谱系障碍。
总之,这些发现确定了一种神经元特异性复合体,该复合体将神经元活动直接与基因组保存耦合在一起,其破坏可能导致发育障碍、神经退行性变和衰老。
研究人员表示,神经元活动对于自适应回路重构至关重要,但在有丝分裂后神经元的长寿命中,对基因组的稳定性构成了固有的风险。神经元是否获得了特殊的基因组保护机制,使它们能够在活动增强期间承受数十年的潜在破坏性刺激,目前尚不清楚。
附:英文原文
Title: A NPAS4–NuA4 complex couples synaptic activity to DNA repair
Author: Pollina, Elizabeth A., Gilliam, Daniel T., Landau, Andrew T., Lin, Cindy, Pajarillo, Naomi, Davis, Christopher P., Harmin, David A., Yap, Ee-Lynn, Vogel, Ian R., Griffith, Eric C., Nagy, M. Aurel, Ling, Emi, Duffy, Erin E., Sabatini, Bernardo L., Weitz, Charles J., Greenberg, Michael E.
Issue&Volume: 2023-02-15
Abstract: Neuronal activity is crucial for adaptive circuit remodelling but poses an inherent risk to the stability of the genome across the long lifespan of postmitotic neurons1,2,3,4,5. Whether neurons have acquired specialized genome protection mechanisms that enable them to withstand decades of potentially damaging stimuli during periods of heightened activity is unknown. Here we identify an activity-dependent DNA repair mechanism in which a new form of the NuA4–TIP60 chromatin modifier assembles in activated neurons around the inducible, neuronal-specific transcription factor NPAS4. We purify this complex from the brain and demonstrate its functions in eliciting activity-dependent changes to neuronal transcriptomes and circuitry. By characterizing the landscape of activity-induced DNA double-strand breaks in the brain, we show that NPAS4–NuA4 binds to recurrently damaged regulatory elements and recruits additional DNA repair machinery to stimulate their repair. Gene regulatory elements bound by NPAS4–NuA4 are partially protected against age-dependent accumulation of somatic mutations. Impaired NPAS4–NuA4 signalling leads to a cascade of cellular defects, including dysregulated activity-dependent transcriptional responses, loss of control over neuronal inhibition and genome instability, which all culminate to reduce organismal lifespan. In addition, mutations in several components of the NuA4 complex are reported to lead to neurodevelopmental and autism spectrum disorders. Together, these findings identify a neuronal-specific complex that couples neuronal activity directly to genome preservation, the disruption of which may contribute to developmental disorders, neurodegeneration and ageing.
DOI: 10.1038/s41586-023-05711-7
Source: https://www.nature.com/articles/s41586-023-05711-7
Nature:《自然》,创刊于1869年。隶属于施普林格·自然出版集团,最新IF:69.504
官方网址:http://www.nature.com/
投稿链接:http://www.nature.com/authors/submit_manuscript.html
